Getting Ready: In our learning activities, we will:
- Observe and record the change of state when substances are heated.
- State the change of state when substances are heated.
- Observe and record the change of state when substances are cooled.
- State the application of change of state of matter in everyday life. We will find out more and share information with members of our families and communities.
- State the change of state when substances are cooled.
- Describe the changes that take place in the water cycle.
- Appreciate that matter changes state when heated or cooled.
- Make candles using candle wax or bee wax.
Activity 5.1: Learning new words
Learn the meaning of these words:
Write the words in your journal or in flashcards.
Change of States of Matter
What do we already know?
From our earlier lessons in Science and Technology, we know matter exists in three states. Name them and give examples in each case.
In our learning activities, we will find out the effect of heating and cooling matter.
Activity 5.2(a): Identifying the various states of matter
In groups, study the pictures labelled 1, 2 and 3. Name the states of matter shown.
Can you change:
- A solid into a liquid?
- A liquid to a solid?
- A gas to a liquid?
How can you do this? What happens when solids are heated? What happens when they are cooled?
What happens to water when you heat it? What happens to water when you cool it?
Identifying the various states of matter
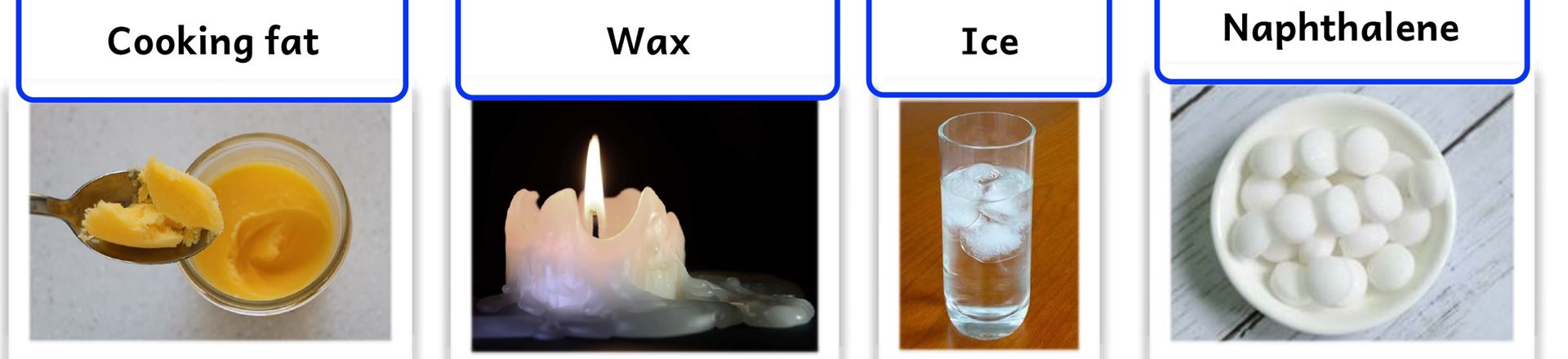
Carry out the following activities. Use the listed learning resources:
- Cooking fat
- Wax
- Ice
- Naphthalene (mothballs)
- Burning candles (or any other source of heat)*
- A metallic spoon (or any shallow transparent container)
- Heat insulator (pieces of cloth or oven gloves)
Activity 5.2(b): Heating and cooling cooking fat, wax or ice
Make these observations at home.
- When food is being cooked, what happens to the cooking fat when the cooking pot is placed on a source of heat?
- What happens to a candle when it is lit and allowed to burn?
Discuss and record your observations. In groups, find out:
- What is the state of cooking fat before the pot is placed on a fire?
- What happens to the fat when the pot is placed on a fire?
- What happens when the fat is removed from the fire and allowed to cool down?
- What is the state of the candle wax before the candle is lit?
- What happens to the wax when the candle is lit?
- What happens to wax when it comes into contact with a cold surface?
- Describe and record the change of state in pictures 1 and 2 above.
- Discuss your findings with your group members.
Safety first: Take care of yourself and others. Use gloves to hold hot objects. Do not touch hot melting solids.
Activity 5.3: Heating and cooling naphthalene
- Work safely in groups.*
- Place some balls of naphthalene (mothballs) in a shallow container.
- Heat the container over a source of heat.
- Hold a bottle or glass tube of cold water above the heated naphthalene.
- Observe what happens.
- Record your observations in a table.
- Discuss the findings with your group members
Material | Change when Heated | Change when Cooled | ||
Cooking fat | ||||
Wax | ||||
Ice | ||||
Naphthalene | ||||
What are the similarities and differences between the behaviour of wax and naphthalene when heated and then cooled?
Promote unity: In what ways will you work with your group members to show respect and care for one another?
Learn more. Grow. Share knowledge with your family and community members
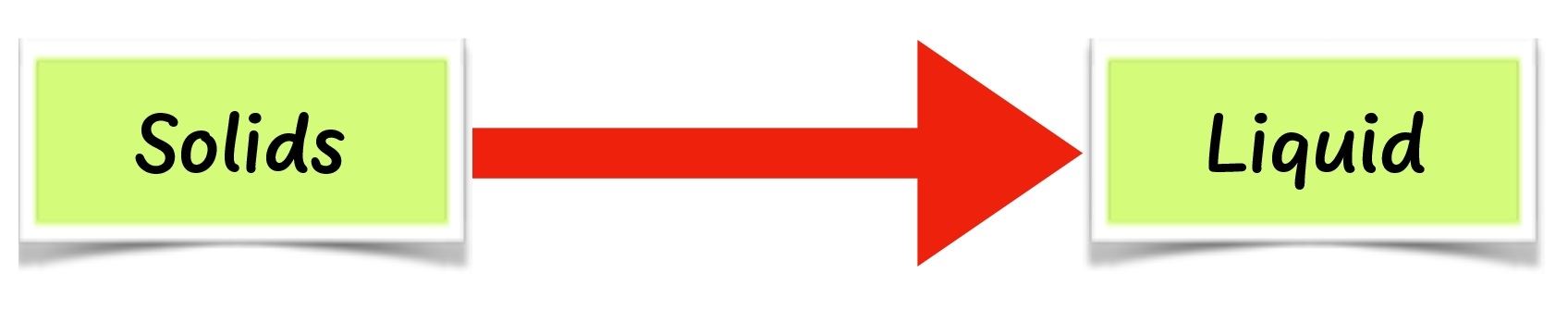
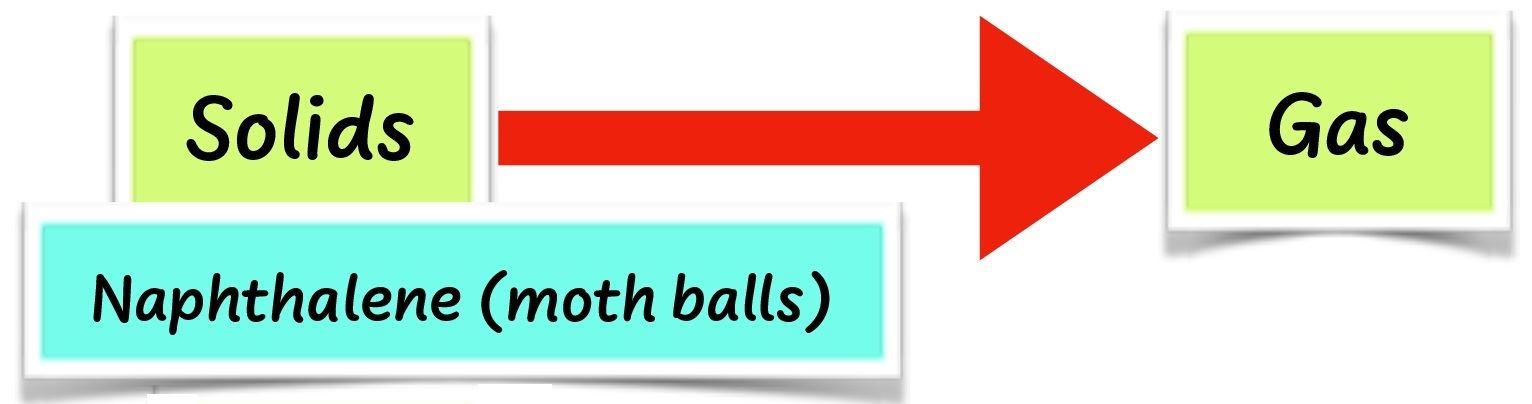
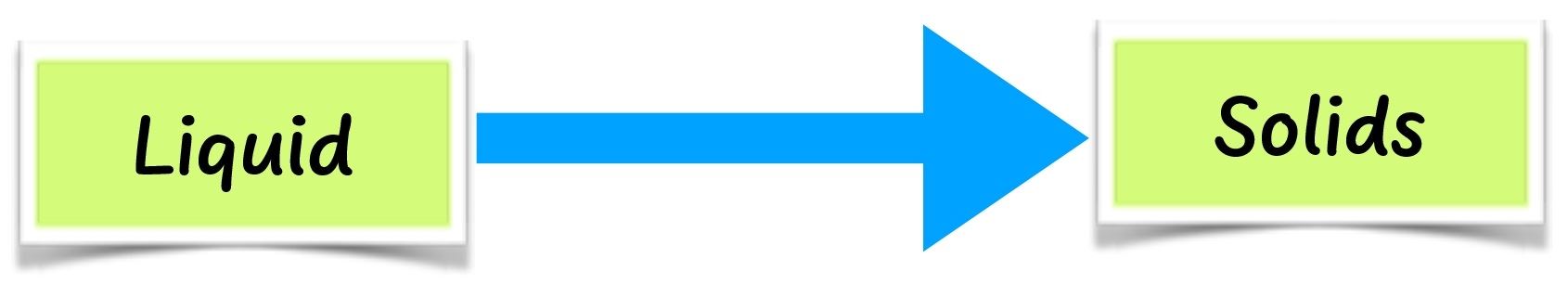
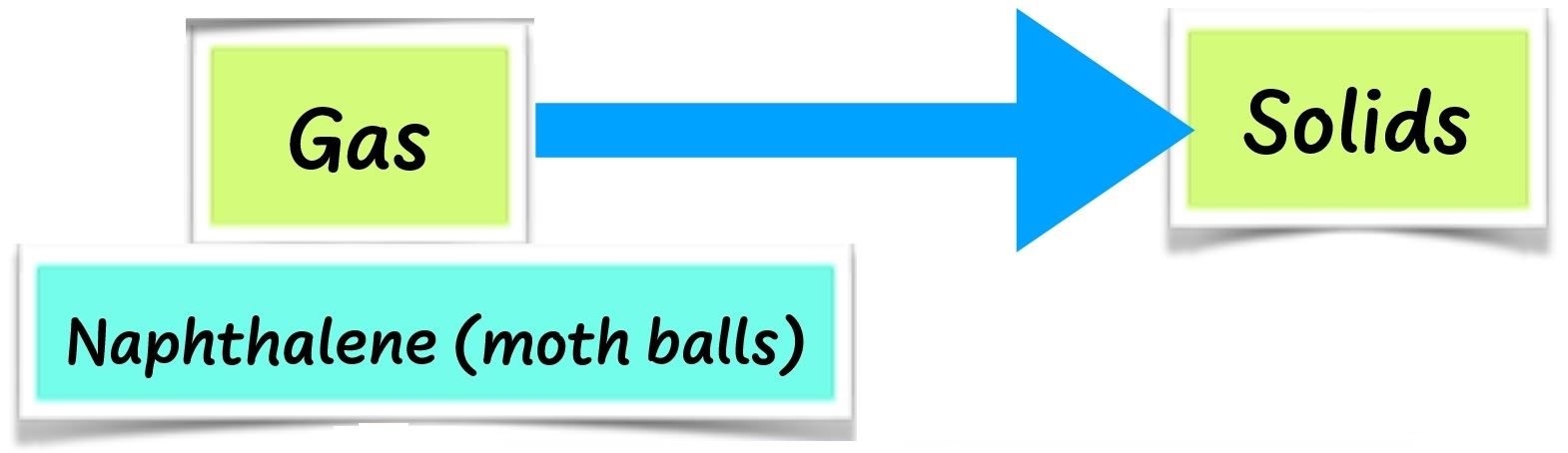
When wax and cooking fat are heated long enough, they change to liquid. This is called melting. When cooled long enough, the liquid changes back to solid. This is called solidification. Some solids such as naphthalene do not melt. They change to gas directly on heating. This is called sublimation.
When cooled, naphthalene changes back to solid without changing into a liquid. When ice is heated, it melts to liquid. This is called melting. When cooled, ice changes to solid ice again. This is called freezing. When solids are heated long enough, they melt. When cooled, they solidify.
Water is normally in liquid state at room temperature. It freezes when cooled under very low temperature.
 |  |
 |  |
Activity 5.4: Discover: The effect of heating and cooling matter
Activity 5.5: Find out more on the effect of heating and cooling matter
Work safely in groups. Discuss pictures 1 to 3. How is change of state used?

Activity 5.6: What happens when liquids are heated and then cooled?
- Work in a group.
- Carry out the activity shown below. You will need these items:
- Water
- A metallic kettle
- A source of heat
- Cold water in a glass bottle or jug
- Heat the water until it boils.*
- Observe what happens as you continue heating.
- Record your observations.
* Observe Safety: Hot water can cause burns and scalds. Be careful. The teacher will guide you to work with the source of heat safely.
- What happens when water in the kettle is heated? What is seen escaping from the spout of the kettle? In what state is it?
- What happens when it comes into contact with the glass jug or bottle containing cold water? Record your observations.
1. Liquid: Change when heated |
 |
2. Gas: Change when Cooled |
 |
Learn more. Grow. Share the knowledge with your family and community members
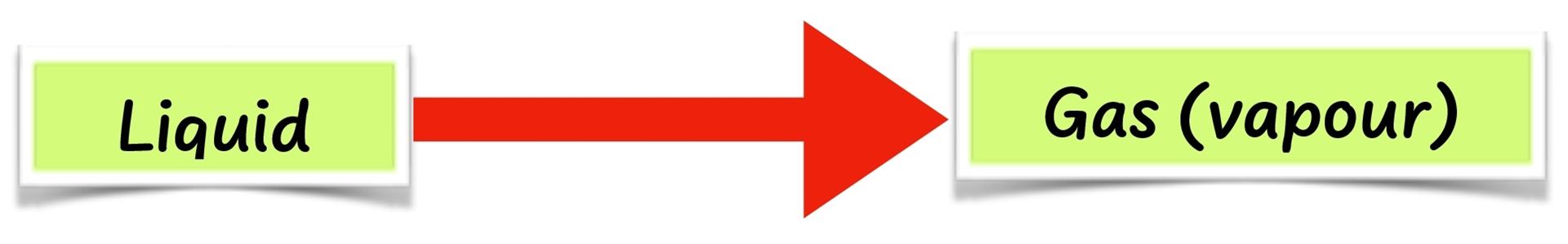
- When water is heated, it changes to vapour, which is a gas. This process is called evaporation or vapourisation.
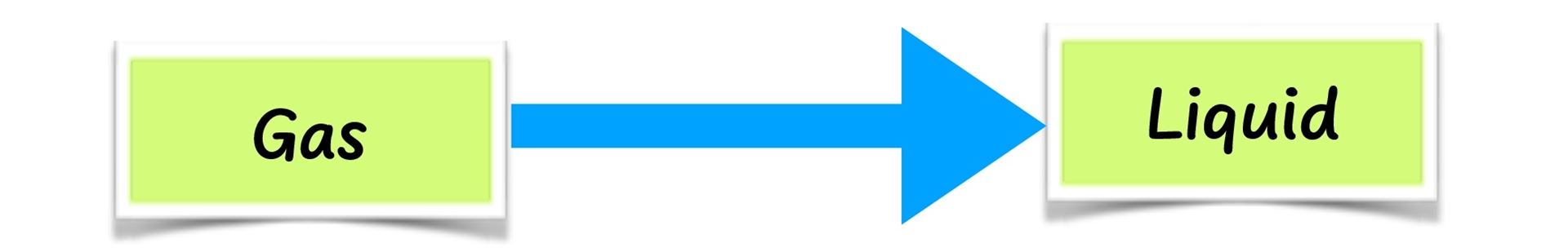
- When the vapour is cooled by the cold water in the glass jug, it changes back to liquid. This is called condensation.
Activity 5.7: What happens when liquids are heated?
Read this story that was written by Riziki’s friend. Explain what happened.
Riziki’s mother poured her bathing water into a metallic bucket. She left it under the sun for sometime for it to get warm. She noticed that the amount of water decreased slightly. Why did that happen? |
Activity 5.8: What happens when water is cooled enough?
- Work in groups. Get a clean ice cube tray.
- Fill all the chambers of the ice cube tray with clean water.
- Place the tray in a freezer and leave it there for about 24 hours.
- Remove the ice cube tray from the freezer after 24 hours and observe. Describe the appearance of the water in the tray.
- Discuss your observations with your group members.
Learn more. Grow. Share the knowledge with your family and community members
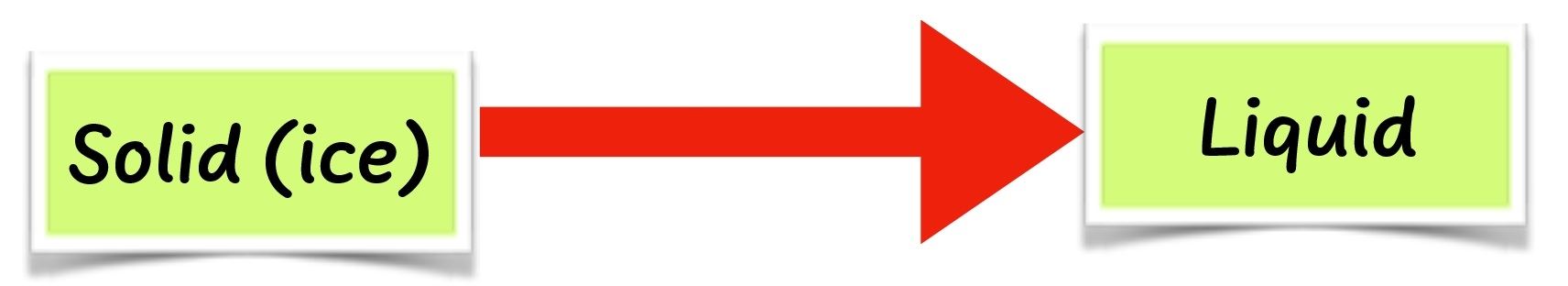
- When water is cooled long enough, it changes to ice. This is water in solid form. This process is called freezing. Substances such as water are normally in liquid state at room temperature. They undergo freezing when cooled long enough to become ice, which is a solid state.
- When the same substances are heated, they melt back to liquid state.
1.  |
2.  |
Activity 5.9: What happens when gases are cooled enough
You will need a mirror. Carry out the activity described below:
- Breathe out onto a mirror.
- Observe what happens to the surface of the mirror.
- Record your observations.
- Discuss the observations with other learners.
Learn more. Grow. Share the knowledge with your family and community members
The air that we breathe out contains water vapour. When it gets into contact with the cold mirror, it changes its state from vapour to liquid. This is called condensation.